Four Key Stops on the Journey from HACCP to HARPC

The Food Safety Modernization Act will soon have a dramatic impact on the food safety and regulatory landscape for facilities producing products regulated by the FDA. The Preventive Controls for Human Foods proposed rule, which is expected to be published as a final rule this month, will significantly change the way food manufacturing companies document their food safety systems. The traditional approach of using the HACCP (Hazard Analysis Critical Control Point) system will transition to the new HARPC (Hazard Analysis Risk-Based Preventive Controls) system. The change will be required by the new FDA regulatory standard defined in 21 CFR 117.26(a).
Food Safety Plan Requirements
FDA regulated facilities will be required to have a written and implemented Food Safety Plan. The Food Safety Plan must include the following contents:
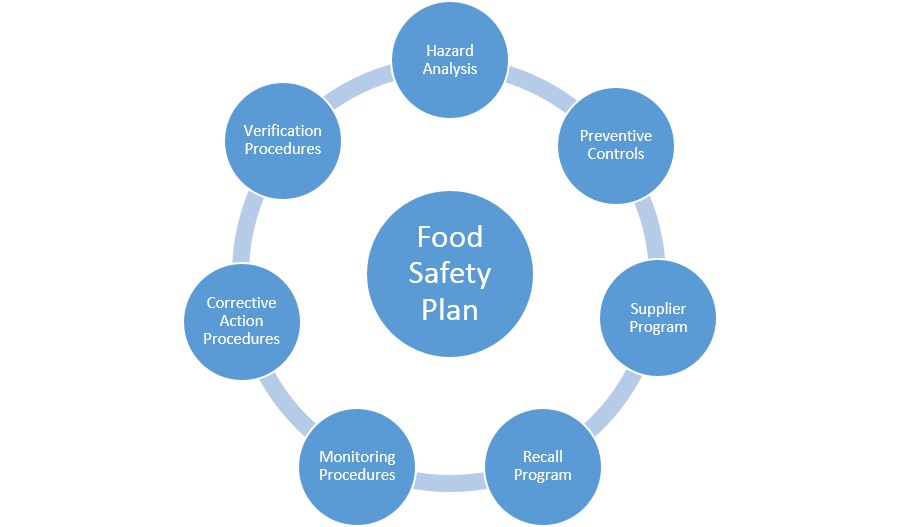
The Food Safety Plan requirements are much more comprehensive than the traditional HACCP plan by itself. A HARPC hazard analysis includes more detailed hazard identification and hazard evaluation
Under HARPC preventive controls encompass numerous prerequisite programs including:
- Process controls
- Allergens
- Sanitation
- Supplier controls
- Recall plan
- GMP’s
- Hygiene training
- AND MORE!
Supplier and recall programs will become formal. Additionally, environmental monitoring as well as corrective and verification procedures will apply not only to critical control points (CCP’s), but to other preventive controls identified and documented by each plant.
As you prepare for the journey to transition from HACCP to HARPC, there are key steps on the way to become prepared and compliant. Focus on the following steps to help you understand and be successful:

Step 1 – Education and Training

The first stop is to take time to educate yourself on the new regulatory requirements and this new approach to your food safety system. The new regulations actually require food safety plans to be developed and reassessed by a “Qualified Individual.”
The proposed rule states a qualified individual must have successfully completed training in the development and application of risk-based preventive controls. Training must be equivalent to a standardized curriculum recognized as adequate by FDA.
It is recommended to attend an accredited HARPC training workshop such as the one offered by Alchemy or others to meet this criteria. The proposed rule does also state that job experience may be used to meet the criteria of a “qualified individual” if such experience includes developing and applying a food safety system, and knowledge at least equivalent to that provided through the standardized curriculum.
There is so much to learn about the regulations and this different approach to food safety that participation in a comprehensive training workshop is highly recommended.
Step 2 – Hazard Analysis
The hazard analysis identified in the 21 CFR 117.130 requirement looks substantially different than the hazard analysis under traditional HACCP Plans. The Hazard analysis is a two-step process: hazard identification and hazard evaluation. The primary differences identified in the proposed rule fall under hazard evaluation.
Hazard evaluation must include an assessment of the potential severity of injury or illness, and the probability that the hazard will occur in the absence of controls. This will require a quantitative risk assessment approach for all hazards identified.
- Radiological hazards must be considered
- Hazards that may be unintentionally introduced must be considered
- Hazards that may be intentionally introduced for purposes of economic gain must be considered
The hazard evaluation phase must also consider the effect of the entire manufacturing process including raw materials, formulation, storage and distribution and sanitation. Even extrinsic factors such as transportation practices and intended or reasonably foreseeable use must be included during this evaluation phase. This approach will require reassessments of existing hazard analysis, and changes the format of documentation. All the required information must be recorded.
Step 3 – Preventive Controls
Preventive controls are applicable to both CCP controls, and many programs often considered to be prerequisite programs. Preventive controls include process controls that are often associated with CCP’s such as cooking, chilling, temperature controls, pH control, and water activity (Aw) control.
Process controls must identify key control parameters including maximum or minimum values (i.e. critical limits or control limits) to be able to assess if the process is in control or not. Process controls specific to a manufacturer’s products, raw materials, processes and environmental factors must also be considered.
At a minimum, documentation of preventive controls required by the proposed rule include:
- Allergens
- Sanitation
- Supplier controls
- Recall plan
- GMP’s
{{cta(‘7fd09bdc-35cf-4b6e-abd1-babdc623d910’)}}
Step 4 – Reanalysis
The Food Safety Plan will be required to be reassessed at the following times:
- Once every three years
- Whenever a significant change is made in activities conducted at the facility
- Whenever plants become aware of new information about potential hazards associated with the food
- After an unanticipated food safety problem occurs
- Whenever a preventive control is found to be ineffective
- Whenever FDA determines it is necessary to respond to new hazards and developments
It will be imperative to document a Food Safety Plan that meets the regulatory requirements and to reassess the plan as often as necessary based on the events above. The food safety plan should always be a living document that accurately represents your processes and controls.
Final Thoughts
HARPC is such a broad topic that there is much more to discuss, so check back for the continuation of this series. We will address the topics of monitoring, corrective actions, as well as verification and validation. Contact Alchemy’s professional services division for more information.
*Editor’s Note: This post is the third in an ongoing HACCP series by Jeff Chilton.






