FDA Labeling Changes: A Survival Guide
Labeling changes can keep you awake at night—literally. Whether it’s an update to a company logo or a minor change to an ingredient statement, changing labels will raise your stress level. Using labels out of sequence, obsolete labels, or just plain wrong labels can be costly. Labeling mistakes will take a chunk out of your bottom line, lead to loss of customer and consumer confidence, or trigger a recall.
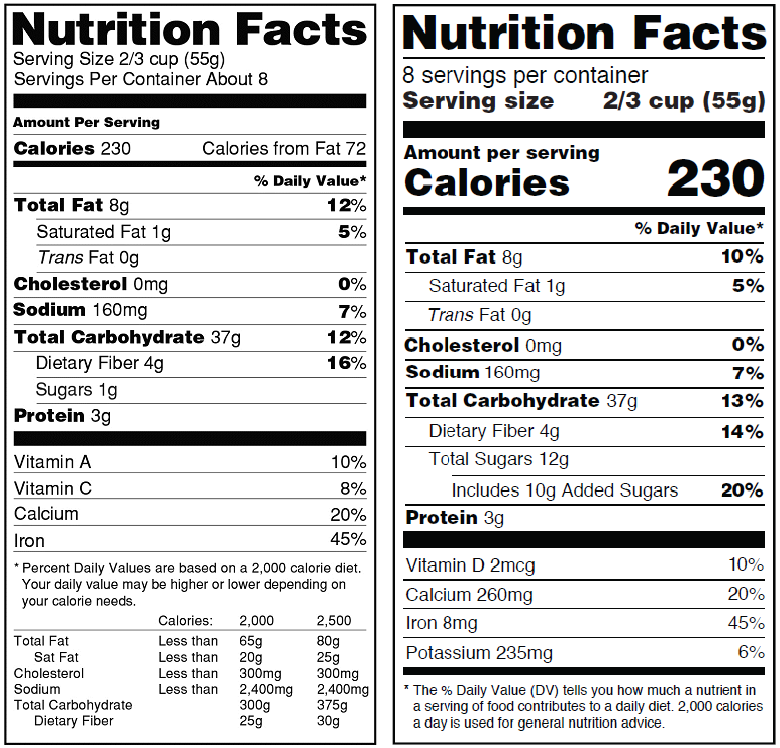
Recently, the FDA published a large change to the requirements for nutritional labeling that is now open for comment. A change like this will affect every package that currently has a nutritional panel. Although it may seem like this change is off in the distant future, it’s never too soon to start preparing for a change of this magnitude. Survival requires a keen awareness of common pitfalls so you can come out on the other end unscathed.
Scope out the Terrain
Reviewing the FDA proposed changes and provide comment—either as an individual, as a company, or through a trade organization. Providing comment is an excellent way to help guide and direct what the finalized requirements will look like. It is a privilege to be invited to provide commentary. Just like casting a vote in an election, failure to participate is a failure to protect your industry’s interests. Let your voice be heard.
Examine the Road Map for a Better Route
Policies and procedures will most likely need to be updated to reflect the changes. A gap analysis done now will provide a good overview of the number of individual changes that need to be made and the scope of the work required. This gap analysis can be quickly updated when the final requirements are published. The work is not wasted and will actually provide a good road map for a head start on work to be done.
Procurement will need to be in the loop if they are responsible for purchasing packaging materials. Now is probably not the time to stock up on labels. There should be a collaborative effort made to ensure labels in inventory are not going to lead to massive stockpiles after compliance dates are set. Be aware of how you’re handling your stock of labels now, and you can avoid having to write off any excess or create unnecessary waste.
Forge Ahead with a Formal Process
Assign a project manager to gather all the information, schedule regular meetings, and bring together all of the appropriate stakeholders—including suppliers. A formal process may include detailed time lines so that printers and packaging suppliers are working together to meet production needs. Gantt charts are an excellent tool for large projects like this. Set regular meetings, keep detailed notes, and outline granular action items, assign responsibilities, determine due dates, and vigorously hold all team members accountable for their respective parts. Everyone needs to participate in the effort.
Use Old Labels First
The old rules of First-In-First-Out (FIFO) or even First-Expired-First-Out (FEFO) are critically important for an overhaul like this. At a plant level it is important to have a process in place to use up old labels first.
There are many ways to accomplish this. Using new item numbers for new packaging, hold tags, and colored tags noting ‘old’ and ‘new’ are just a few of the examples that may be employed for segregation. Choose wisely when revamping or developing a process by involving the frontline worker. Frontline workers live it and breathe it and will provide valuable insight into what works and what doesn’t.
Enable Success with the Right Tools
The key to success of any program will be to ensure that all employees have a complete understanding of what is happening, what their role is, and why it is important. Accountability goes hand in hand with any training, so develop a robust program, fully train the workforce, and enforce strict adherence. Exceptions are a slippery slope, and once the downward slide begins, the integrity of the program will be compromised.
Scrutinize Everything Leaving Nothing to Chance
As new labels are received, be sure they are reviewed by a subject matter expert to ensure any printing errors or wrong versions are not received as good inventory. Pull samples from more than one pallet to provide reassurance that old inventory from the packaging supplier is not mixed in with new versions. It’s better to scrutinize the materials as they are received, before they are included in inventory. Notify the appropriate departments immediately of any discrepancies as there may be consequences to sister facilities making the same products or mix-ups in order quantities that will affect scheduling.
{{cta(‘bc964694-0f8c-4b0f-bd73-7619172eb583’)}}
Out with the old and in with the new.
As old labels are depleted from inventory and new labels begin to be received, the programs should kick into high gear. Tracking old inventory becomes more important than ever. Counting and recounting may become a daily requirement just to keep production on schedule. Switching over may not be as easy as using up the old and then putting the new on the line. There must be a way to track products for recall and traceability purposes. Planning up front will allow a manufacturing plant to plan for traceability and make changes with as little disruption as possible.
Reduce your risk.
Don’t leave obsolete packaging languishing in the warehouse; it will be waiting for someone to accidentally bring it to the line to be used. As soon as it becomes obsolete, place it on hold, placard the material, wrap it in red tape, pull it out of usable inventory, and make arrangements to discard. The longer obsolete material sits around, the easier it will be to forget about it and inadvertently pull it to the line.
Plan for the next time.
Once the transition is complete, be sure to do a debrief. Pull all stakeholders together to discuss what worked well and what needed improvement. Don’t forget the importance of celebrating the accomplishments. Having detailed notes will provide a thorough survival guide for the next round of changes. After all, change is inevitable!






