Prepare Now for BRCGS Issue 9 Audits in February

Recently the Brand Reputation Compliance Global Standards (BRCGS) organization announced Issue 9 of its global standard for food safety. It’s always a major event when any of the Global Food Safety Initiative (GFSI) owners update their standards, which happens every three years. It’s important to take time to learn the new requirements to help prepare your company for audits that start in February.
These measures can help you maintain a world-class food safety and quality assurance system and help prevent non-conformances during upcoming audits. BRCGS has done a great job in updating the standard itself while providing a thorough amount of guidance and information to help you meet their requirements.
For those companies already certified to Issue 8, it will make it easier to step up to this latest update.
I want to take a moment to provide a brief overview and background on Issue 9, and invite you to watch a more comprehensive discussion with a replay of our recent webinar: BRCGS Issue 9: Your Keys to Preparation.
Why the Change?
You might be asking why were these changes introduced and what are their objectives? One of the biggest reasons for Issue 9 was to encourage a broader understanding and greater emphasis on the importance of a food safety culture. Even though this issue was included in Issue 8, the organization wanted to emphasize further the need for a positive food safety culture and offer guidance on how to achieve it.
The second objective is to help ensure the BRCGS Global standard for food safety is consistent with both the Codex General Principles of Food Hygiene and that it meets GFSI’s benchmarking requirements.
In addition, BRCGS wanted to expand the audit options to include the use of information and communication technology (ICT); update the requirements associated with core product safety activities, such as internal audits, root cause analysis, preventive actions and incident management; and improve clarity for sites completing animal primary conversion and producing animal feed.
Issue 9 and its Timeline
Issue 9 standards and the guidelines were published in August for everyone to review. Approved Training Partner training will begin in September. In October, training will be available to the auditors and sites. Audits based on Issue 9 will begin February 1st. Alchemy Consulting does have ATP’s on staff and offers Issue 9 Sites Training virtually or in person if we can be of assistance with your training needs.
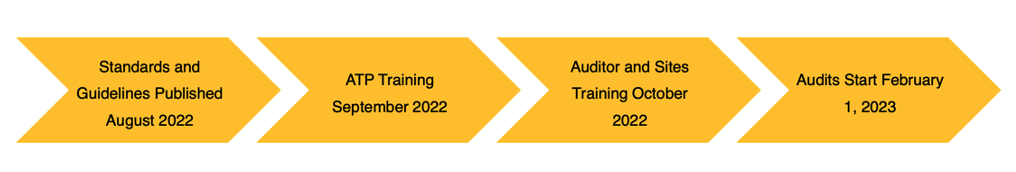
Any audits occurring up to January 31 will be to Issue 8. And then every audit occurring after February 1st will be to Issue 9.
Audits will come in three forms:
- Announced Audits – that are scheduled in advance. This will be a full onsite audit both of the documentation and facility conditions.
- Blended Audits – that include a remote offsite review of documentation and an onsite facility inspection.
- Unannounced Audits – that are full mandatory inspections every three years. It is acceptable to schedule blackout periods for up to 10 days. Also, the certification body will notify you within 90 days of the audit date of that window.
Examples of What’s New in Issue 9
With that background, let’s take a look at some of the key changes in Issue 9. This blog will touch on some of the new changes from a high level, citing five sections. For a deeper dive, watch a replay of our recent webinar: BRCGS Issue 9: Your Keys to Preparation. I’ll list these changes below by their standard identifications.
Food Safety and Quality Culture Plan 1.1.2
To a degree, the need for a food safety culture was already part of the BRCGS in that it requires you to communicate your food safety culture to employees and give them a means provide feedback. That focus remains with some additional requirements including a way to define specific activities for communication, training, feedback, behaviors and performance measurements. It also includes a requirement to show that action plans are continually maintained. Food safety culture assessment surveys are a great way to check the pulse of your organization. However, they are not required by the standard.
Senior Management Objectives – Authenticity 1.1.3
When it comes to this standard, notice how the term “authenticity,” is used in several different clauses of the standard. “Authenticity” is now used in place of “integrity.”
By the standard’s definition, authenticity ensures food or raw materials purchased and offered for sale are of the nature, quality and substance expected. In regard to objectives, one of the things that we commonly see is that companies don’t do a great job of defining their senior management objectives, the KPIs that you should identify as part of your food safety and the quality system itself.
This standard requires monthly meetings be held to address food safety, legality, authenticity and quality topics. A confidential reporting system must be established and communicated to employees so they can report concerns related to food safety, legality, authenticity and quality. And senior management must provide human and financial resources to produce safe, legal, authentic products to specified quality.
Management Reviews 1.1.4
Once your objectives are defined, management reviews are the best way to drive continuous improvement of your food safety and quality system. According to the standard, the management review meetings must evaluate the effectiveness of your food safety and quality culture plan.
The review process should evaluate:
- Previous management review action plans and timeframes.
- The results of internal, second-party and/or third-party audits.
- Any unmet objectives and why. This information will be used for setting future objectives and to facilitate continual improvement.
- Any customer complaints and the results of any customer feedback.
- Any incidents including both recalls and withdrawals, corrective actions, out-of-specification results and non-conforming materials.
- The effectiveness of the systems for HACCP, food defense and authenticity, and the food safety and quality culture plan, and your required resources.
HACCP – Food Safety Plan – Section 2
This section references multiple standards that involve validation, verification, reviews, flow diagrams and prerequisite programs. So, I’ll just call it “section 2.” This section demonstrates updates that were created to align with Codex Alimentarius HACCP principles. Sites are advised to avoid multiple plans with different terminology. HACCP plans should be reviewed prior to any changes that might affect food safety. Food safety teams must verify flow diagrams annually and whenever changes occur. And PRP terminology must change from “sanitizing” to “disinfecting.” In addition, the annual review of the food safety plan should be a comprehensive and well documented process.
Internal Audits 3.4
This section also requires a Food Safety and Quality Culture to also be covered in your internal audit program. Quarterly audits are still required, and open product areas necessitate audits on a monthly basis. The updated standard requires all activities that form a part of a site’s food safety and quality systems, be covered at least once a year. This includes anything relevant to food safety, authenticity, legality, and quality.
The scope of the internal audit program must include HACCP or food safety plans, prerequisite programs such as hygiene and pest management, food defense and food fraud prevention plans, and procedures implemented to achieve the Standard.
These are just five of 20 updates we cover in the webinar, which will help explain the changes in greater detail. If you have questions about Issue 9 and how to prepare for audits in February, please contact us. We have team of food safety consultants who are ready to help.





